- 2026
- 2024
- 2023
- 2022
- 2021
Annex 1 and the impact on the cleanroom wipes selection

Since August 2022 it’s now published in its final version – the new Annex 1, part of the EU- GMP guidelines for aseptic manufacturing. A revision was urgently required to adapt the existing guidelines to the new technologies and methods. This new version comprises a total of 58 pages with 11 chapters for a considerably extended scope of application. The following sentence is particularly interesting here “…. includes additional areas (other than sterile products) where the general principles of the annex can be applied”.
The importance of Quality Risk Management (QRM) is now even more clearly emphasized and seen as the basis for justifying possible deviations - supported by a corresponding risk assessment.
This then leads us directly to the requirement to implement a “Contamination Control Strategy” (CCS) as a comprehensive concept for contamination control across the entire manufacturing area.
Central aspects of this documented Contamination Control Strategy include:
- inter alia the system design,
- the premises and equipment,
- the personnel,
- the cleaning and
- disinfection and the monitoring systems - to name just a few.
But how can cleanroom wipes be classified here?
They are not explicitly mentioned in the new Annex 1 but are of course part of every cleaning procedure and therefore they are subject to a corresponding risk analysis and assessment.
It should be noted that the primary goal of cleaning is to remove contamination without introducing new contaminants through this process or the materials used for it. In order to be able to carry out a proper risk assessment here, however, it is first necessary to precisely determine the individual requirements:
- What are the contaminants to be removed?
Particles, microorganisms, endotoxins, ions...?
- In which working environments should the wipes be used?
ISO class, GMP class, Isolator, RABS?
- Which chemicals are used with the wipes?
Detergents, disinfectants?
- Are there any additional requirements?
Packaging (e.g. VHP Barrier Bag)
Packaging size
Wipe size
Wipe count/packaging unit
- Are there quality certificates available or other documentations?
- Are audits necessary/possible?
Once all these requirements have been defined, the next step is to select a wipe that meets these requirements.
Is it sufficient for this and for the subsequent risk analysis to select and compare wipes based on a data sheet?
We at Texwipe say: NO - that's definitely not enough! At best, it can provide a first orientation, but more is needed for a proper risk assessment.
For example - what do you know about cleanroom wipes and the effect of material, type of fabric and manufacturing process on the level of cleanliness and quality? Let's make a small digression on this.
Let's start with the material or substrate. In general, it can be said that plastic substrates are “cleaner” (i.e. generate less particle) than natural ones such as cellulose or cotton. But there are also major differences within the plastics and plastic families
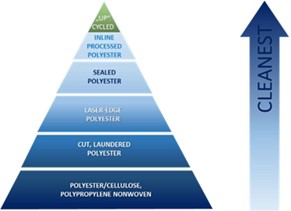
The next point is the type of fabric – how the substrates are processed.
The pyramid shows that knitted polyester proves to be "cleaner" than e.g. fleece fabric (nonwoven). In addition to that, the possible edge sealing plays a major role in minimizing particle release.
But the manufacturing process also plays a role that should not be neglected and you will usually not learn anything about this in the available data sheets.
Texwipe is the only manufacturer worldwide who has developed and uses the 3 manufacturing processes with different levels of automation - further described below.
Although all processes take place in certified cleanrooms, increasing automation naturally goes hand in hand with a reduction in biological contamination caused by humans.
Nevertheless, most of the cleanroom wipes produced worldwide are still manufactured conventionally in a very manual process, in which the wipes are washed and dried in conventional industrial machines after cutting. And the downstream manufacturing steps (e.g. stacking, packaging) are done by employees more or less manually.
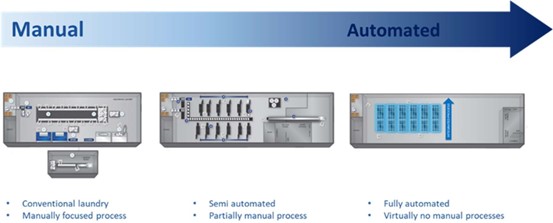
As early as the 1990s, Texwipe developed the Vectra-Process - a semi-automated manufacturing. Above all, the washing and drying procedure was automated and changed in such a way that the particle generation from wipes produced in this way could be reduced even more. Due to less employees in the cleanroom and especially in the area in front of the packaging, less biological contamination can also be expected here.
But as in all areas of pharmaceutical production and related industries, the focus at Texwipe is on further automation and the first fully automated wipe production was developed with the Vertex process. From washing up to the final packaging in sealed bags, the wipes are manufactured here in a micro-cleanroom - which is placed in an also certified cleanroom - without human intervention. This globally unique manufacturing process allows Texwipe to consistently offer low-particle and bioburden-reduced wipes in our standard portfolio.
But sometimes even that is not enough for very special requirements. It may then be necessary to develop customer-specific products that contribute to contamination control and therefore to risk minimization even better than any standard wipes.
It is certainly a great advantage that Texwipe was able to set up a local production site in the EU (Netherlands), which makes it possible to develop such customer-specific products in close cooperation with European drug manufacturers.
And - Of course, all sterile products, whether from the standard portfolio or customer- specific, are validated, documented, and tested for endotoxins
Like all Texwipe operations, the one in the Netherlands is ISO 9001:2015 certified and works according to a Global Quality System. Similar to the GMP guidelines, this includes an effective Quality Risk Management and a Contamination Control Strategy. Of course, on a different level than in the aseptic manufacturing of medicinal products, but in principle they are quite comparable.
In summary, one can say that the risk assessment of a cleanroom wipe required according to EU-GMP is only possible if you define your own requirements precisely and then, with knowledge of materials, manufacturing processes and quality criteria, look for the most suitable product - either from the standard portfolio or, if there is nothing suitable there - a customer-specific wipe that is developed in a joint project.
If you have more questions, contact us.
