- 2026
- 2024
- 2023
- 2022
- 2021
70% Ethanol Percentage Level in a Denatured Alcohol Solution
Texwipe’s Denatured Alcohol solution is a blend of USP-grade Ethanol, USP-grade Isopropanol (isopropyl alcohol, IPA) and USP-grade purified water. This solution is submicron filtered, filled into cleaned containers, and double-bagged. Texwipe offers several gamma-irradiated products containing 70% Denatured Ethanol solution. All products are lot coded with an expiration date for easy recordkeeping. Bottles come fully assembled and ready for use. The adjustable spray head offers stream delivery or coarse spray.
70% Denatured Ethanol solutions are intended to be used for cleaning and residue removal:
- Cleaning general surfaces
- Pre-cleaning before disinfectant application
- Removing residues after disinfectant application
- Cleaning gloved hands
- Wiping down items for pass-through to controlled environments
Bottles of Denatured Ethanol solution are sometimes used over several days. It is important to know if the concentration of the Ethanol in the solution is varying as it is used.
The U.S. Pharmacopeial Convention (USP) contains a denatured ethanol monograph, Rubbing Alcohol. The denaturing chemicals used in this monograph are acetone, methyl isobutyl ketone, and sucrose octaacetate or denatonium benzoate. These chemicals are added to make the ethanol unsuitable to drink by making the ethanol poisonous or unpalatable; otherwise, the alcohol beverage tax is applied and must be paid. Formulation 23-H denatured alcohol with added sucrose octaacetate and denatonium benzoate would leave significant residue on surfaces and the acetone and methyl isobutyl ketone may also chemically attack, e.g., haze, soften or dissolve, common plastics, e.g., Plexiglas®, found in cleanrooms.
Because of the residue and chemical issue, USP-grade IPA was chosen as the denaturant for the USP-grade ethanol to make a denatured ethanol solution. Water is added to this solution at a level to make a 70% volume of ethanol in an IPA and water solution. The denatured alcohol portion of the formulation follows Formula No. 3-C from 27 CFR 21.37 (a) of Subpart D - Specially Denatured Spirits Formulas and Authorized Uses. These components are available in USP-grade, do not leave residues that other denaturing formulations could, and are chemically innocuous toward plastic surfaces.
Purpose
Since Texwipe’s Sterile 70% Ethanol in Denatured Alcohol products are offered meeting the USP Rubbing Alcohol Monograph volume percent ethanol (68.5% to 71.5%), it is important to document the ethanol concentration stability as a function of time. A study was implemented to monitor the ethanol concentration over 28 days after the bottle was opened to simulate in-use conditions. A recommended in-use shelf-life is intended to assure the appropriate quality of 70% Denatured Ethanol throughout its use by demonstrating compliance with USP’s Monograph for Rubbing Alcohol volume percent ethanol.
Experimental
Three 16-oz bottles of Texwipe’s Sterile 70% Denatured Ethanol TX3267 and three 32-oz bottles of 70% Denatured Ethanol TX3265 were set aside for the study and were kept under laboratory conditions, room temperature (20°C/68˚F) and pressure (one atmosphere). The specific gravity was measured using a Mettler-Toledo International, Inc. (Columbus, OH) Densito 30PX on the first, seventh, fourteenth, and twenty-eight days after opening the bottle. The meter displayed the specific gravity at 25°C/25°C conditions. As part of the study, each sample bottle was sprayed six or seven times each day throughout the study. The specific gravity for each bottle was measured twice on the indicated test days.
Results
Specific gravity is a unitless number which is the ratio of the density of the substance and the density of water. Since density and the specific gravity are affected by temperature, the conditions at which the densities were measured are specified. The typical format is in the style of a fraction, e.g., 25°C/25°C. The left value references the temperature for the substance, and the right value references the temperature for the water.
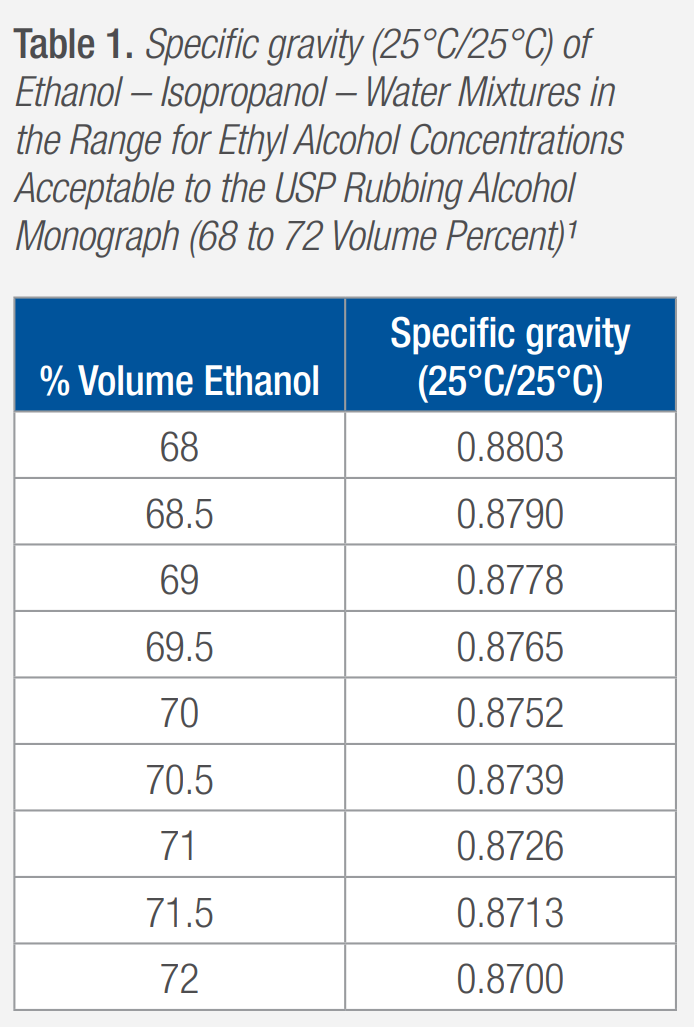
Specific gravity is commonly used to determine the concentration of substances in solutions. The concentration of the ethanol in the ethanol – isopropanol – water solution was determined by locating the measured specific gravity in a table and referencing the percent volume (% Volume) in the denatured ethanol solution with that specific gravity. The specific gravity and its corresponding percent volume denatured ethanol used for this study are compiled in Table 1.
The periodic specific gravity (25°C/25°C) test results for the study are compiled into Table 2. The specific gravity for each bottle was measured twice on each test day. In Table 2, the first measurement result of the specific gravity is indicated with an “R1” in the Sample Number and Measurement column. The second result is indicated with an “R2.” The % volume result is determined through the use of the specific gravity–percent volume data found in Table 1.
Discussion
The two specific gravity values measured for a specific bottle and day are repeatable. The values were identical. For each bottle, the specific gravity did not change from the first day (Day 1) to the last day (Day 28) of the study. The overall specific gravity values complied with the range (68.5% to 71.5%) found in the USP Rubbing Alcohol Monograph2.
Conclusion
The study showed that the specific gravity and the corresponding % volume of ethanol did not change for six bottles measured over 28 days under in-use conditions (repetitive sprays each day of the study duration). The percent volume values complied with the range found in USP’s Monograph for Rubbing Alcohol
For further information and images click here.