- 2026
- 2024
- 2023
- 2022
- 2021
Texwipe Sterile Wipes and Swabs
Sterile wipers were developed by Texwipe to clean sterile environments quickly and easily, without compromising the sterile nature of those environments.
Although wipers are not medical devices, ITW Texwipe has chosen to follow the guidelines of the Association for the Advancement of Medical Instrumentation (AAMI) concerning the validation of radiation sterilization and the requirements of the United States Pharmacopeia for endotoxin testing.
Sterilization, Validation, Documentation
Wipers are typically sterilized by gamma irradiation, electron beam, or steam autoclaving. Table 1 summarizes the features of gamma irradiation and electron beam sterilization. Texwipe has chosen gamma irradiation over other sterilization techniques for the following reasons:
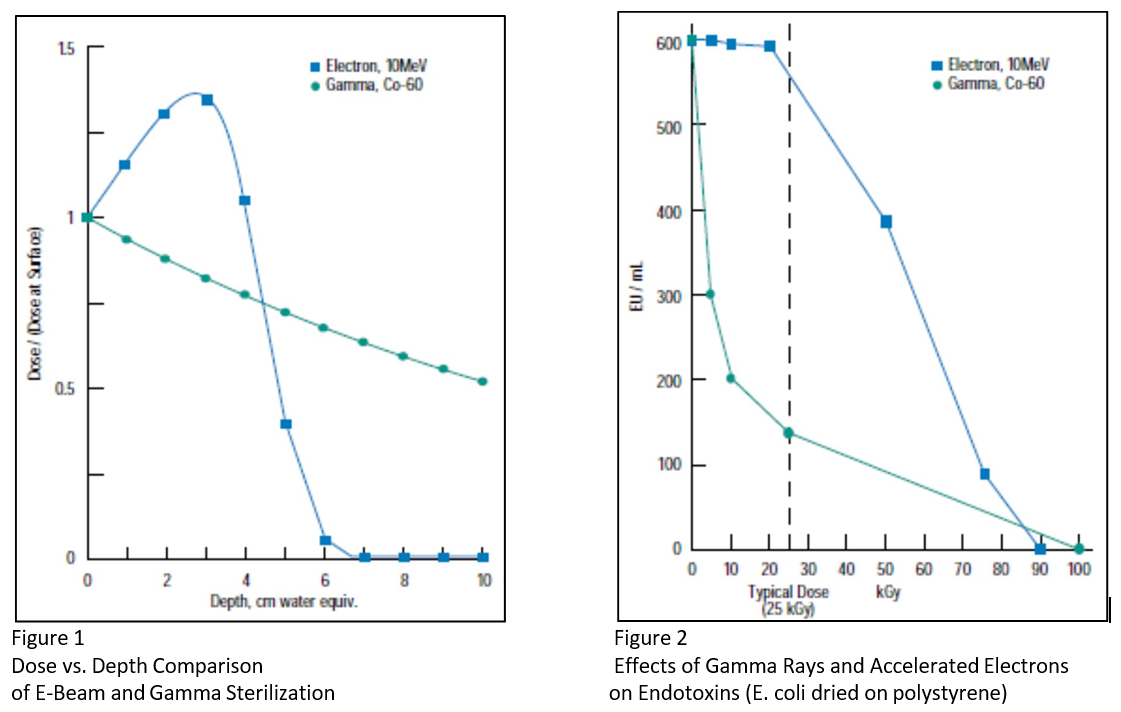
- Gamma irradiation has more penetrating power than an electron beam. Figure 1 illustrates this point.
- Gamma irradiation is a relatively easy process to validate. This is especially important because Texwipe sterile products adhere to AAMI standards.
- Gamma radiation leaves no residues from treatment.
- Gamma radiation is compatible with many of the wiper materials and solutions used in Texwipe products.
- Gamma irradiation has been shown to reduce endotoxin levels to a greater extent than electron beam irradiation.
(Bacterial endotoxins, or pyrogens, are fever-causing materials from the outer cell membranes of Gramnegative bacteria. Steam autoclaving does not reduce endotoxin levels. Figure 2 depicts the reduction of endotoxin levels after gamma and electron-beam irradiation2)
Comparison of Gamma and Electron-Beam Irradiation Techniques for Sterilization (Table 1)
|
Feature |
Gamma (cobalt-60 source |
Electron-Beam |
|
Penetration |
Greater |
Less (energy dependent) |
|
Field Uniformity |
More uniform |
Less uniform |
|
Dose Uniformity |
More uniform |
Less uniform |
|
Dose Rate |
Slower |
Faster |
|
Experience |
More |
Less |
|
Sterilization |
Equal |
Equal |
|
Validation |
Simpler |
More complex |
|
De-pyrogenation |
Better |
Worse |
Figure 1 (below) compares the normalized dose versus depth for a product irradiated by electron beam at 10MeV energy with another irradiated by gamma rays from a cobalt-60 source. This graph illustrates the more limited penetration of the electron beam and is valid for most plastics and objects with the density of water. Electron beams penetrate only about 6 cm of water and about as deeply into 70% isopropyl alcohol /30% water solutions since the densities are about the same. The dose from gamma irradiation is 60% of the surface dose at the depth where the electron beam dose is virtually zero.
Figure 2 (below) quantifies the reduction of endotoxin levels. At the typical irradiation dose of 25 kGy, the levels of e. coli endotoxin, as measured by Guyomard et al2, were reduced by almost 80% when irradiated by gamma rays and only 10% when irradiated by an electron beam.
Validation Process Overview
Since Texwipe sterile products are sterilized according to AAMI guidelines for the validation of radiation sterilization, a strict processing regimen is followed. Wipers are tested for bioburden and the data are corrected for recovery. A sub-dose validation is performed to ensure that there are no unusually radiation-resistant bacterial strains present. Once the sub-process dose validation is achieved, the products are gamma-irradiated at the dose determined by the bioburden and confirmed by the sub-process dose validation. All irradiated product is supplied with a Certificate of Processing.
Pyrogen Testing
Bacterial endotoxins, or pyrogens, are fever-causing materials from the outer cell membranes of Gramnegative bacteria. Most bacteria in clean water are Gram-negative. When the bacteria die, endotoxin is
released into the water. An example of Gram-negative bacteria that we are all familiar with is e.coli, often found in contaminated water. Minute levels of these bacterial toxins are associated
with certain bacterial diseases and the production of fever, shock, and death in humans and animals.
Removing pyrogens from contaminated samples is extremely difficult. Sterilization techniques kill living organisms, and since pyrogens come from dead cells, standard sterilization techniques, with few
exceptions, are not effective in reducing pyrogen levels. Prevention of bacterial contamination is the best course of action to ensure highquality products. Therefore, it is essential to ensure that the materials used during the production of the parenteral drug or medical device do not contribute to the pyrogen levels.
The allowable limits of endotoxins in human and animal parenteral drugs, biological products and medical devices are established by the FDA. Texwipe SterileWipes and Sterile Flexpacks conform to the
requirements of the United States Pharmacopeia 23. This limit, applied to devices in contact, either directly or indirectly, with the cardiovascular system or lymphatic system, has been set at less than 20 endotoxin units/device (<20 EU/device).
Test Method
The widely used assay method for bacterial endotoxins is based on the blood cell extract, or amebocyte, from the horseshoe crab (Limulus polyphemus). Much like a human donates blood, the horseshoe crab "donates" some of its blood, which is broken down into its components, plasma and amebocytes. The LAL (Limulus amebocyte lysate) is produced by lysis (rupture) of these amebocytes, and the LAL reagent is a mixture of proteins and salts whose origin is the amebocyte. The LAL test is an effective measure of endotoxin levels as it is extremely sensitive. The typical gel clot test is capable of detecting endotoxin levels as low as 0.03EU/mL (about 0.003ng/mL or 0.003 parts per billion).
As outlined in USP 23, the test method includes the following steps:
1. Between three and ten wipers are extracted with LAL Quality Water (USP Water for Injection).
2. A standardized LAL reagent is mixed with the test samples and heated.
3. A firm gel is formed if the concentration of endotoxin exceeds the reagent labeled sensitivity.
Further, a validation assay is performed in the following manner:
1. The reagent is tested against an endotoxin standard, with serial dilutions.
2. The extracts are tested to assure they do not inhibit or enhance the formation of the gel when
endotoxins are present.
3. This validation assay is performed in quadruplicate.
Test Results
Although wipers are not considered medical devices and are not required to conform to USP 23 standards, the test results in Table 3 indicate that Texwipe Sterile products do meet a standard of <20 Endotoxin Units/wiper.
Endotoxin Test Results for Wipers (Table 3)
|
Product Name |
Substrate |
Endotoxin Level |
|
SterileWipe HSII (TX3210) |
Polyester-cellulose blend |
< 20 EU/wiper |
|
SterileWipe LP (TX3211) |
Polyester |
< 20 EU/wiper |
|
SterileWipe LP10 (TX3212) |
Sealed-edge polyester |
< 20 EU/wiper |
|
Sterile PolySat (TX3213) |
Polypropylene pre-wetted with 70% IPA/30%DIW |
< 20 EU/wiper |
|
Sterile TechniSat (TX3214) |
Polyester-cellulose blend pre- wetted with 70% IPA/30% DIW |
< 20 EU/wiper |
|
Sterile Wipe AS 10 (TX3215) |
Sealed-edge polyester |
< 20 EU/wiper |
|
Sterile PolySat (TX3216) |
Polypropylene pre-wetted with 70% IPA/30%DIW |
< 20 EU/wiper |
|
Sterile TechniSat (TX3217) |
Polyester-cellulose blend pre- wetted with 70% IPA/30% DIW |
< 20 EU/wiper |
EU = Endotoxin unit
IPA = Isopropyl alcohol, DIW = Deionized water
Conclusion
Texwipe Sterile wipers are irradiated to a probability of Non-Sterility (also called Sterility Assurance Level) of 10–6 in accordance with AAMI standards and are tested for pyrogen levels per USP 23. This ensures the highest quality of wiper products available for cleaning and disinfection of equipment and environmental surfaces under sterile conditions. These products are ideal for cleaning pharmaceutical aseptic filling areas, sterile suites, prep rooms, microbiological laboratories and biotech manufacturing facilities.